Whether you’re sipping coffee on a cold morning or cruising down the highway in your car, you’re interacting with thermal systems every day. These systems are responsible for managing heat—how it’s generated, transferred, and used. But what exactly is a thermal system, and where do we encounter them in our daily lives?
Let’s explore the fascinating world of thermal systems through real-world examples that make the science behind them easy to understand and appreciate.
What Is a Thermal System?
A thermal system refers to a physical system that involves the transfer, transformation, or storage of heat energy. It includes components like:
- Heat sources (like a flame or the sun)
- Heat sinks (areas or materials that absorb heat)
- Fluids (air, water, oil, refrigerants)
Insulators and conductors
These systems rely on principles from thermodynamics—the study of heat and energy flow. The basic goal of a thermal system is to move heat from one place to another or to convert it into useful work.
Real-World Thermal System Examples
Now that we have the basics covered, let’s dive into some common and practical examples of thermal systems you likely encounter regularly.
Home Heating Systems (Furnaces and Radiators)
One of the most familiar thermal systems is the home heating system. Whether it’s gas, electric, or oil-powered, its job is to generate and distribute heat.
How it works:
- A furnace burns fuel to generate heat.
- This heat is transferred to air or water.
- The warm air or water is circulated throughout your home using ducts or pipes.
- Thermostats help regulate temperature.
- This system relies heavily on convection (heat transfer through fluid movement) and conduction (direct heat transfer through materials).
Refrigerators and Freezers
- Yes, even cooling appliances are thermal systems! A refrigerator removes heat from inside the fridge and releases it into your kitchen.
How it works:
- A refrigerant absorbs heat from the food compartment.
- It travels through coils to the compressor.
- The compressor increases pressure, heating the refrigerant.
- The heat is released into the room as the refrigerant cools and condenses.
- Despite being cold appliances, they are excellent examples of closed-loop thermal systems using heat pumps.
Air Conditioners
Similar to refrigerators, air conditioning units are thermal systems designed to cool spaces by removing heat.
How it works:
- They extract warm air from inside.
- A refrigerant captures the heat and transports it outside.
- The cooled air is then circulated indoors.
- Modern HVAC (Heating, Ventilation, and Air Conditioning) systems combine both heating and cooling functions, making them complex yet efficient thermal systems.
Automobile Engines
Your car’s internal combustion engine is a powerful thermal system.
How it works:
- Fuel combustion generates heat.
- This heat causes gas expansion that moves pistons.
- The pistons turn the crankshaft, moving the car.
- A cooling system (usually using water or coolant) prevents overheating.
- In this system, chemical energy is converted into thermal energy, which then becomes mechanical work. It’s a prime example of thermodynamics in motion.
Solar Water Heaters
Harnessing the sun’s energy, solar thermal systems heat water for household use.
How it works:
- Solar collectors (usually on the roof) absorb sunlight.
- The heat warms up a fluid inside the collector.
- The fluid transfers heat to the water in a storage tank.
- This eco-friendly thermal system reduces reliance on fossil fuels and demonstrates radiative heat transfer—where energy is transferred through electromagnetic waves.
Thermos Flasks
A thermos is a simple but effective thermal system designed to minimize heat transfer.
How it works:
- It has a vacuum layer between two walls.
- The vacuum prevents conduction and convection.
- Reflective surfaces reduce radiation.
- Whether you’re keeping coffee hot or water cold, thermos flasks slow down heat loss or gain—showing how insulation plays a vital role in thermal systems.
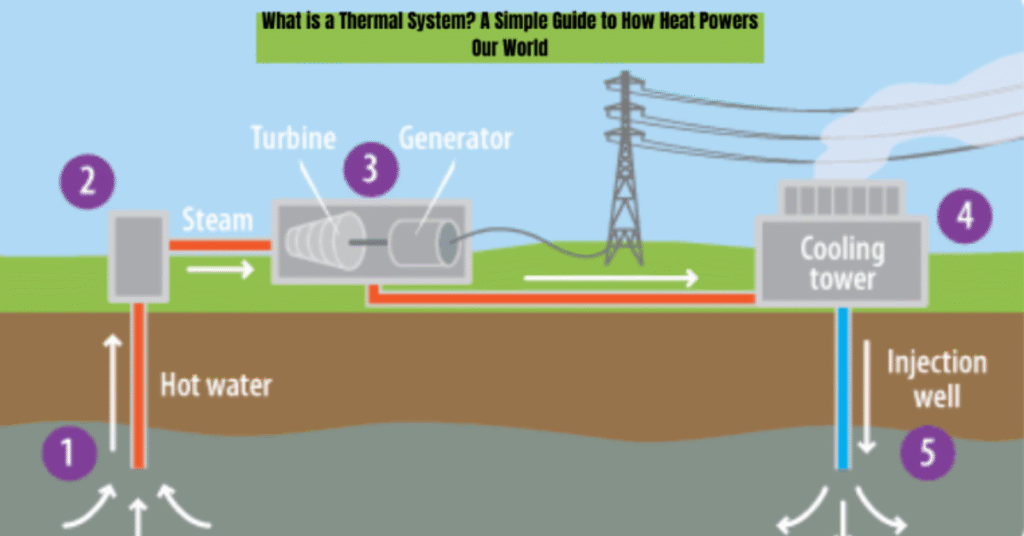
Steam Power Plants
Used for electricity generation, steam power plants are large-scale thermal systems.
How it works:
- Water is heated in a boiler to produce steam.
- The high-pressure steam drives turbines.
- The turbines rotate generators that produce electricity.
- The steam is cooled and condensed back to water for reuse.
- This is a textbook example of thermal energy being converted to mechanical energy, then to electrical energy.
Cooking Appliances (Ovens, Stoves, Microwaves)
- Cooking is full of thermal system activity.
- Ovens use conduction and convection to bake food.
- Gas stoves burn fuel to transfer heat directly via conduction.
- Microwaves use radiation to excite water molecules and heat food.
- Each appliance operates as a thermal system tailored to specific cooking needs.
How We Design and Optimize Thermal Systems
- Thermal systems are everywhere, but they must be carefully designed and managed for maximum efficiency and safety. Engineers consider:
- Heat transfer rate
- Energy losses
- Material properties
- Environmental conditions
- For example, car engines are equipped with radiators and fans to manage heat. Buildings use insulation and reflective coatings to control internal temperatures.
- The goal is often to reduce wasted heat or reuse it—leading to innovations like waste heat recovery systems, thermal batteries, and heat exchangers.
Why Thermal Systems Matter
- Understanding thermal systems helps us:
- Improve energy efficiency (in homes, vehicles, industries)
- Reduce environmental impact (through better fuel usage and less waste)
- Enhance comfort and safety (with climate control and reliable heating/cooling)
- In a world facing climate change and rising energy demands, optimizing thermal systems is more important than ever.
Conclusion
Thermal systems are all around us, quietly powering our lives through the transfer and management of heat. From simple household appliances like ovens and thermoses to complex systems like car engines and power plants, they demonstrate the essential role of thermal energy in both everyday comfort and industrial processes. Understanding these systems not only helps us appreciate the science behind them but also encourages smarter energy use and innovation. As we face growing environmental and energy challenges, improving and optimizing thermal systems will be key to building a more efficient and sustainable future.